Portal site for Food-related Business Operators » For Those Who create labels on Processed Foods in the Japanese Language for the First Time
[For Those Who Create Labels on Processed Foods in the Japanese Language for the First Time]
- Explanations on points that require your special attention among the food labeling rules different from rules of other countries -
When selling foods domestically in Japan, labeling on containers and packages in the Japanese language is mandatory.
Any food product that does not comply with proper labeling goes against the Food Labeling Act and therefore cannot be sold.
There are also detailed rules on how to prepare the contents of labels.
Please comply with the rules stipulated in the Food Labeling Act (Food Labeling Standard) to avoid the lack of or prevent missing any required item in the label.
This also applies to importing and selling foods manufactured overseas.
≪Labeling example≫ This food is manufactured in country A, and then imported to Japan.
 |
|
≪Points that require your special attention because of differences from rules of other countries≫
(1) “Allergen Labeling”
In Japan, if any of the allergens that are designated by the government that may cause food allergies are used in the ingredients, labeling the allergen in the “Ingredients” and “Additives” columns is mandatory. In addition to inspecting the use of secondary and tertiary ingredients, any risk of unintentional contamination in the manufacturing processes should be included as a check in the examination procedures. In some cases additives may contain allergens that cause food allergies.
Alcohlic drinks are not required to carry allergen information.
Since each country specifies allergens which are subject to mandatory allergen labeling differently, special attention is required (see the table below).
(Labeling rule)
When any ingredient of a food item contains an allergen, display the name of the allergen in the parentheses immediately following the ingredient. The label must be written as“
If an additive derives from an allergen, display the name of the allergen in the parentheses immediately following the name of the substance. The label must be written as“
(Labeling example)
| 原材料名 | 小麦粉、砂糖、マーガリン、チョコレートチップ(乳成分を含む)、卵、食塩 |
●Foods for which allergen labeling is mandatory in Japan (8items)
Shrimp/Prawn, Crab, Walnuts,Wheat, Buckwheat, Egg, Milk, Peanuts
  |
●Foods for which allergen labeling is recommended in Japan (20 items)
Almonds, Abalone, Squid, Salmon roe, Oranges, Cashew nuts, Kiwifruit, Beef, Sesame, Salmon, Mackerel, Soybeans, Chicken, Bananas, Pork, Matsutake mushrooms, Peaches, Yams, Apples, Gelatin
|
Gluten-free labeling |
| According to the standards of US, EU, and other countries, a food that contains gluten below 20 ppm is allowed to be designated as “gluten-free.” On the other hand, the Japanese allergen labeling rule requires the wording “contains wheat” as allergen labeling in case the food contains more than a few ppm of the total amount of wheat protein. Please note that a food labeled as “gluten-free” may be required for allergen labeling for wheat according to the total amount of wheat protein. |
(2) “Foods that contain aspartame”
In Japan, foods that contain aspartame are subject to mandatory labeling of “contains L-phenylalanine compound” on containers and packages.(Labeling example)
| 添加物 | 甘味料(アスパルテーム・L‐フェニルアラニン化合物) |
(3) “Foods containing designated ingredients, etc.”
In Japan, foods that contain Coleus Forskolin, Celandine, White Kwao Krua, Black Cohosh are subject to mandatory labeling of “Foods containing designated ingredients, etc.” Other ingredients may be added in the future.(Labeling example)
| 名称 | プエラリア・ミリフィカ粉末含有食品 |
| 原材料名 | 乳糖(国内製造)、プエラリア・ミリフィカ粉末、… |
| 添加物 | 加工デンプン(小麦由来)、セルロース、… |
| 内容量 | 10g |
| 賞味期限 | 20××.11.30 |
| 保存方法 | 直射日光、高温多湿を避けて保存 |
| 販売者 | 株式会社〇〇 東京都〇〇区〇〇1-2-3 03-0000-0000 |
| 製造所 | 株式会社□□
東京都□□市□□町1-2-3 |
|
|
Ingredients designated by the Minister of Health, Labour and Welfare based on Article 8, paragraph 1 of the Food Sanitation Act | As of February 5, 2024 |
| Name of designated ingredients, etc. | Common names or other names in the market |
| コレウス・フォルスコリー | Coleus、Forskolin、Coleus forskohlii |
| ドオウレン | クサノオウ、ハックツサイ、ヨウシュクサノオウ、グレーターセランディン、Celandine、Greater celandine、Swallow-wort、Chelidonium majus |
| プエラリア・ミリフィカ | 白ガウクルア、White Kwao Krua、Pueraria mirifica |
| ブラックコホシュ | ラケモサ、Black cohosh、Black snakeroot、Actaea racemosa |
(4) “Date labeling and preservation method”
In Japan, in terms of the consumption period for unopened foods, foods in which the quality may deteriorate easily and rapidly are subject to the mandatory wording of “Expiration Date (better not to be consumed after the date),” while other foods that may have a longer duration period are subject to the mandatory wording of “Best Before (till the date the quality is guaranteed)” in the Date labeling column, in principle, using the year-month-day order. This is not the date of manufacture. In addition, the preservation method that guarantees the consumption period should also be included.(Inappropriate labeling example observed often for imported products)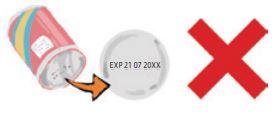 |
(Correct labeling example)
|
|
Labeling example of date labeling |
| Western calendar (YYYY.MM.DD) | Japanese calendar | Examples |
| 2025.07.21 | 令和6年7月21日 | 2025.7.21 令和6年7月21日 06.07.21 |
| 2026.07.21 | 令和7年7月21日 | 2026.7.21 令和7年7月21日 07.07.21 |
(5) “Ingredients, Additives”
In Japan, ingredients and additives are differentiated and subject to mandatory labeling in the ingredients and additives columns respectively. In principle, the general name is used for ingredients while the name of substances is used for additives. Since each country has a different labeling method, attention is required (see the table below).
In addition, please check the Food Sanitation Act because some additives are prohibited for use in foods for sale in Japan.
(6) “Nutritional Components Labeling”
In Japan, in addition to showing the name on the label, ingredients, additives, the expiration date, and the preservation method, and listing of nutritional components is mandatory. For ingredients such as water or spices that provide little contribution as a source of nutrition or for foods for sale by small business operators that meet criteria stipulated by the Consumption Tax Act or the Small and Medium-sized Enterprise Basic Act, the nutritional components labeling may be omitted.
● Nutritional components listing should be written in the Japanese language. Even when an imported product has a nutritional components labeling, it is required to have wording in the Japanese language in compliance with the Food Labeling Standard.
| (Nutritional Components Labeling example in a foreign language) | (Nutritional Components Labeling example in the Japanese language) | |
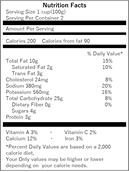 |
→ | 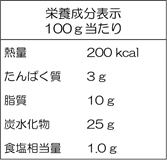 |
● The five items, namely, “Calories,” “Protein,” “Fat,” “Carbohydrate,” and “Sodium (displayed after converting to the salt equivalent weight) are mandatory labeling items in the nutritional components labeling, and should be labeled in this order. Since there are also other rules in the labeling method such as labeling other items of nutritional components that are not subject to mandatory labeling (e.g. Calcium, Vitamin C) below the salt equivalent weight, your confirmation is required.
|
Labeling Sodium |
| For nutritional components labeling in other countries, it is common to label Sodium as the “Sodium (amount)”, however, for nutritional components labeling in Japan, it is required to show the amount of Sodium as “the salt equivalent weight” after converting to the salt equivalent. ≪Converting formula from Sodium to the salt equivalent weight≫ Salt equivalent weight (g) = Sodium (mg) × 2.54 ÷ 1,000 |
● When labeling specific nutritional components on the containers or packaging in Japanese language such as “Full of Vitamin C” or “Sugarless” in an emphasized manner, this applies to “Emphasizing Nutrients Labeling” which is required to meet designated criteria. Having such labeling in a foreign language(s) on imported foods does not necessarily mean that the same wording can be used in the Japanese language. Please confirm the standards for the emphasizing nutrients labeling.
● In the nutritional components labeling, values based on the evidence is required. Please confirm with the distributor or request a test at an analytical institution on food components in Japan as needed to show the appropriate values.
The information above explains only some parts of the rules that require special attention.
There are some other rules in labeling which may differ from other countries.
When making a label, please confirm by referring to the “Food Labeling Act.”
≪Material that you need to confirm when making a label≫
- Food Labeling Act, the Ministry of Justice
- Food Labeling, the Consumer Affairs Agency
- Explanation on the “Window into Food Sanitation”, Tokyo Metropolitan Government (available only in Japanese language)
- For Those Who Create Labels on Processed Foods in the Japanese Language for the First Time, Tokyo Metropolitan Goverment (japanese)
The labeling requirement also applies for sale of fresh foods such as vegetables, meats, and fishes, or additives.
In addition, the food labeling may be related not only to the Food Labeling Act but also to other laws and regulation such as the Act against Unjustifiable Premiums and Misleading Representations, the Measurement Act, the JAS Act (the Act on Standardization and Proper Quality Labeling of Agricultural and Forestry Products), and the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices. Please also confirm the application of these laws.

